The Zeeman Effect
The Zeeman Effect is the splitting of spectral lines on two or more components with similar frequencies for a case of a light source in magnetic field. It was first observed in 1896 by the Dutch physicist Pieter Zeeman on yellow sodium D-lines when a flame was placed between strong magnetic fields. Discrete splitting of spectral lines to more than 15 components was discovered several years later.
Zeeman was awarded with The Nobel Prize for physics in 1902. He shared it with his supervisor Hendrik Anton Lorentz, also a Dutch physicist. Lorentz already had his own ideas about light influenced by magnetism and so he developed a theory that electrons circling around a nucleus oscillate and emit electromagnetic radiation.
Magnetic fields are able to influence these oscillations and the frequency of emitted radiation. This theory was verified by Zeeman's experiments and later it was refined and generalized by quantum mechanics. Quantum theory explains what happens during the transition of an electron from one discrete energy level to other. The electron's potential energy changes and the difference is compensated by emission or absorption of a quatum of light with the same energy.
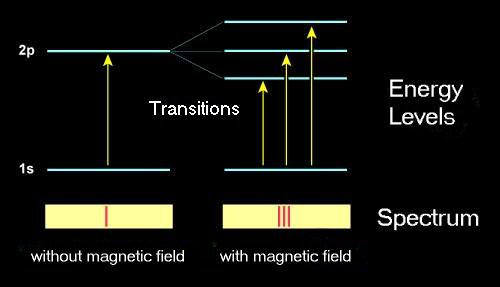 You can find detailed quantum explanation for a hydrogen atom here (website of The Johns Hopkins University).
You can find detailed quantum explanation for a hydrogen atom here (website of The Johns Hopkins University).